Is it just me, or is there a slightly diabolical look on this young woman’s face? This picture appeared in the October 1937 issue of Popular Science, meaning that it was probably taken right around the time of the Hindenburg disaster.
Is it just me, or is there a slightly diabolical look on this young woman’s face? This picture appeared in the October 1937 issue of Popular Science, meaning that it was probably taken right around the time of the Hindenburg disaster.
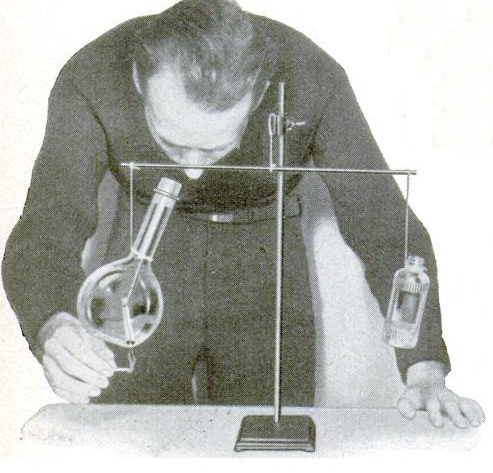
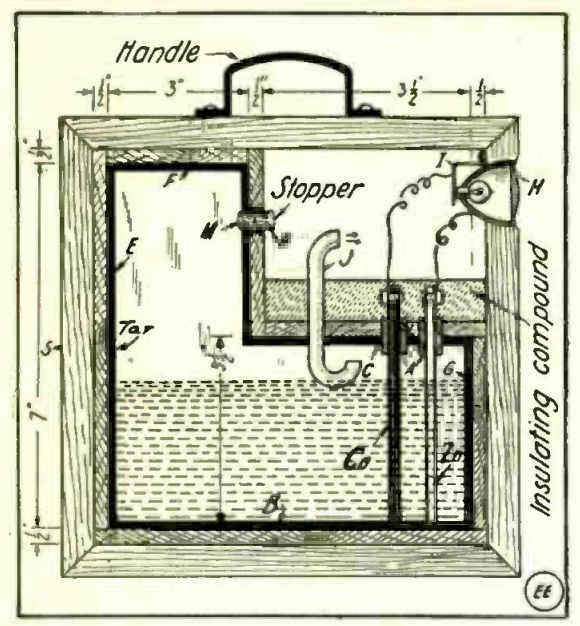
She is preparing what was probably the first place experiment in the 1937 science fair by filling soap bubbles with hydrogen gas. The magazine carried a number of scientific experiments involving air pressure. Most were very safe, even by our modern standards. But instead of settling for those completely safe experiments, she decided to generate some hydrogen gas. Normally, soap bubbles fall, because they’re heavier than air. But by blowing soap bubble with hydrogen gas, they rise to the ceiling.
Hopefully she didn’t try to relax by lighting up a Camel while doing the experiment, since the magazine (perhaps with the Hindenburg fresh in the memory) warned that the experiment should be “kept safely away from open flames.” The hydrogen gas is easily generated in a flask or bottle containing scraps of zinc. To make the hydrogen, you simply add a little bit of sulphuric or hydrochloric acid. This 10% HCl toilet bowl cleaner will probably do the trick. You should be able to find some inexpensive item made out of zinc at the hardware store. If you can’t, you can probably use galvanized nails, or just buy a small piece of the metal.
 If your parents or teacher are uncomfortable with you playing with dangerous chemicals and explosive gasses, then the article includes some other less dangerous experiments. Perhaps you can start with one of these to convince them that you use good lab practices.
If your parents or teacher are uncomfortable with you playing with dangerous chemicals and explosive gasses, then the article includes some other less dangerous experiments. Perhaps you can start with one of these to convince them that you use good lab practices.
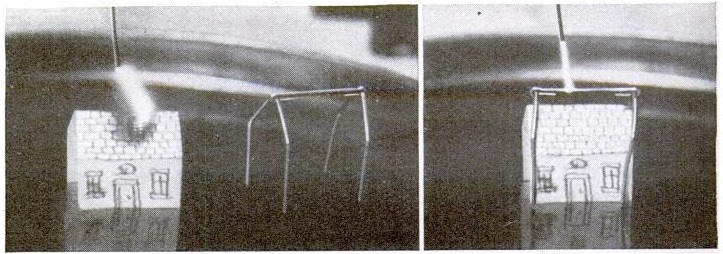
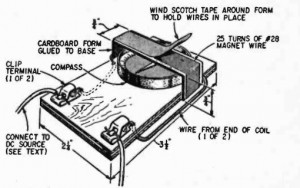
For example, shown at the left is a simple experiment showing the effects of air pressure. You first inflate a balloon inside a bottle. The magazine didn’t tell you exactly how, since they realized that kids 80 years ago could figure it out themselves. You can also figure it out yourself, but just in case you can’t, simply insert the uninflated balloon into the top of the bottle, blow it up part way, tie it off, and then poke it in.
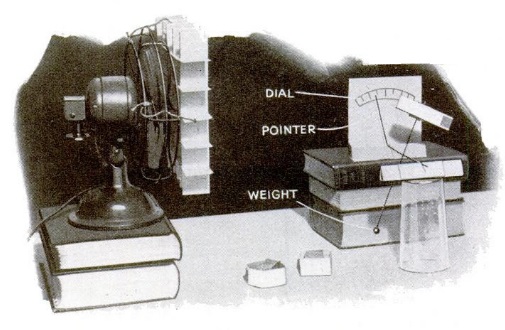
 Once the balloon is inflated, you seal up the bottle and either blow air in or suck air out. The balloon will expand and contract depending upon the pressure in the bottle. The same general principle is used to construct the barometer shown here. If the barometer isn’t quite sensitive enough to respond to changes in barometric pressure (or if you’re impatient), then you put it inside a larger container and blow in or suck out air to make the barometer show the changes in pressure.
Once the balloon is inflated, you seal up the bottle and either blow air in or suck air out. The balloon will expand and contract depending upon the pressure in the bottle. The same general principle is used to construct the barometer shown here. If the barometer isn’t quite sensitive enough to respond to changes in barometric pressure (or if you’re impatient), then you put it inside a larger container and blow in or suck out air to make the barometer show the changes in pressure.
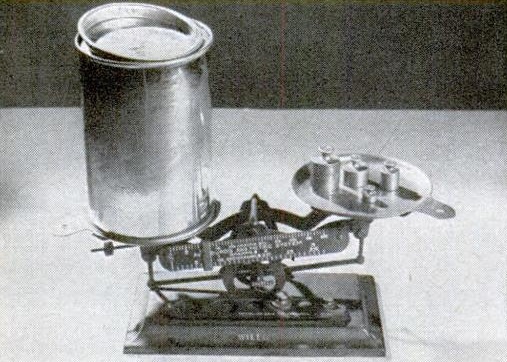
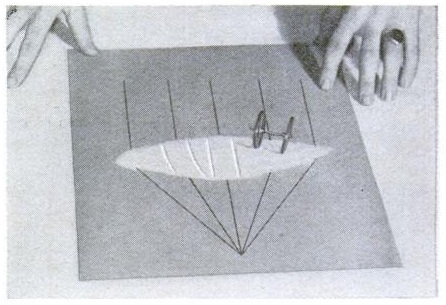
Finally, the magazine showed how to weigh air, using a system similar to what we previously showed for weighing smoke. As shown below, you put a little bit of boiling water in a jar and firmly seal the lid. As the water cools, the steam in the jar condenses, leaving a partial vacuum.
After it cools, you carefully weigh the jar. Then, you loosen the lid, to allow air to rush in. The total weight increases, and the difference is the weight of the air.