When the aspiring young mad scientist is looking for ideas for the science fair, someone invariably suggests making a homemade battery. Making a battery is a fairly simple proposition. All you need are two dissimilar metals and an electrolyte. A common choice for electrolyte is a mild acid such as lemon juice, and copper and zinc make good dissimilar metals. No matter how badly you construct the thing, a little bit of electrical current is bound to flow, and you can probably coax a little bit of light out of a light-emitting diode (LED) or even power a small electronic device such as a digital clock.
In fact, for students with limited scientific abilities, you can just go out and buy yourself a Potato Clock kit
. You simply open the box and jab the electrodes into a potato, and the potato juice serves as the electrolyte. It’s completely safe, since I can hardly think of any chemical more benign than potato juice. If you drop the potato on the floor, you don’t need to bother calling the haz mat team. And unless you screw up horribly, the clock will instantly come to life. There’s nothing wrong with the humble potato clock, but if you’re reading this looking for ideas, you probably want to come up with something a bit more spectacular. And while you’re at it, you probably want to use chemicals slightly more dangerous than potato juice.
So you might want to go back in history a bit when adults weren’t quite so concerned with hazardous chemicals, and use something slightly more powerful in making your battery. You can go back in time fifty years, when adults let their responsible children play around with slightly more dangerous chemicals such as household bleach, often referred to by its most popular brand name, Clorox. Not only will you have more fun, but you’ll wind up with a much more powerful battery, suitable for powering much bigger electronic devices.
For details on how to put the battery together, you can go to page 98 the Fall 1965 issue of Elementary Electronics. That article describes two batteries that you can make at home, both of which are hundreds of times more powerful than the one running that other kid’s potato clock.
Warning: Bleach really is a dangerous chemical. You need to be careful with it, and keep it out of the reach of children who are not as smart as you are. If you get any on your clothes, your mom will be mad. If you get any in your eyes, you’re facing a major medical emergency. Your mom is probably right when she tells you, “you can put an eye out with that.” Ask your parents and/or teacher for permission. If they balk at the idea, ask them to read the article about how to make the battery. To show how responsible you are, show them that you read the Material Safety Data Sheet (MSDS).
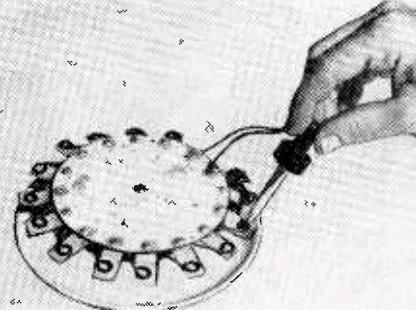
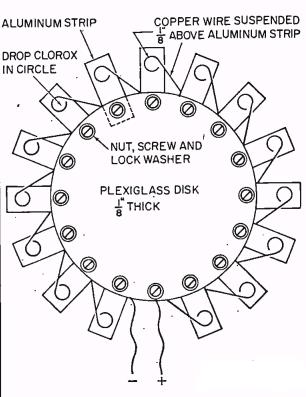
The article shows how to make two batteries. The first one, while much more powerful than the potato, is “more of a novelty than a practical device.” It is shown here, and consists of fifteen sets of aluminum strips and copper wires. The metallic pieces are arranged in a circle around a piece of Plexiglas. The copper and aluminum are close together, but not touching. When ready for use, a drop of bleach is placed on each one. When the last drop of bleach is added, the connected radio or other device springs to life. If measured with a voltmeter, the complete battery will put out about 15 volts. However, this drops when there’s an actual load, and 15 cells is about right to power a radio that normally calls for 9 volts.
Unlike the potato battery, this one will run a radio for several minutes. But the article concedes that it’s more of a novelty. Therefore, the article goes on to describe another more powerful battery. The bigger one is even suitable for use around the house in case of a power outage. If the power is out and you’ve used up the last battery, there’s probably still a bottle of bleach down in the laundry room, good for hundreds of homemade batteries.
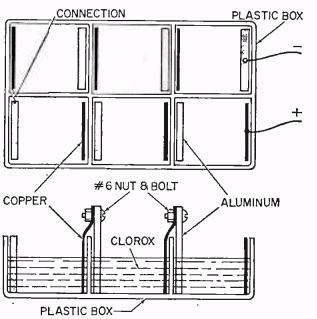
The larger battery is constructed in a plastic ice cube tray. You use six of the individual compartments, so you can cut the ice cube tray in half and make two batteries. Each compartment of the tray contains one piece of aluminum and one piece of copper. You simply fill each compartment with bleach, and you have enough power to run a radio for several hours. When the battery finally goes dead, you pour out the old bleach and replace it. You can re-use the battery hundreds of times before the aluminum finally gets worn away completely.

Voltaic pile similar to the 1799 version. Wikipedia photo.
With either battery, you have essentially recreated the work of Allesandro Volta, who invented the Voltaic pile in 1799. He was eventually able to build a battery large enough to administer an uncomfortable electric shock. Until the electric generator came along in the 1870’s, anything that required electricity (such as the telegraph or telephone) was powered by batteries similar to those created by Volta.
Armed with this fifty year old article, a bottle of bleach, and a few pieces of scrap metal, you can now make your own Voltaic pile. You’ll get to use dangerous chemicals. You can generate significant amounts of electrical power. Perhaps you can even administer uncomfortable electric shocks to your friends, teachers, and parents.
You’ll have the most interesting project at the science fair. And the kid who goes home with a participation ribbon for his potato clock is going to be pissed.
Check out my other science fair ideas, some of which are slightly dangerous.
Click Here For Today’s Ripley’s Believe It Or Not Cartoon
![]()


Pingback: 1916 Homemade Flashlight | OneTubeRadio.com
Pingback: 1967 Homemade Galvanometer | OneTubeRadio.com
Pingback: 1968 Instant Battery | OneTubeRadio.com