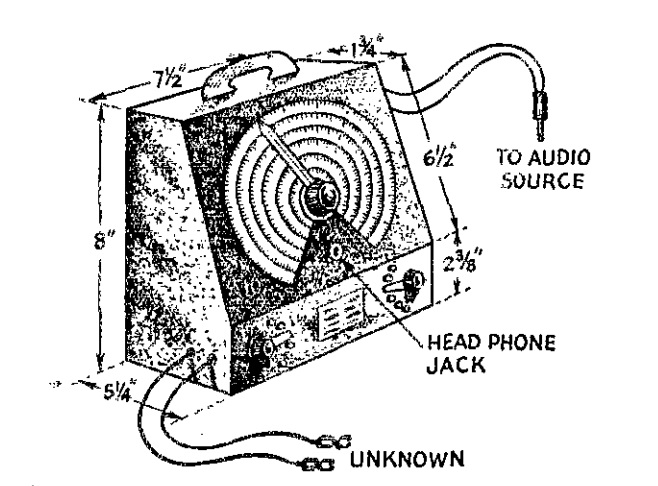
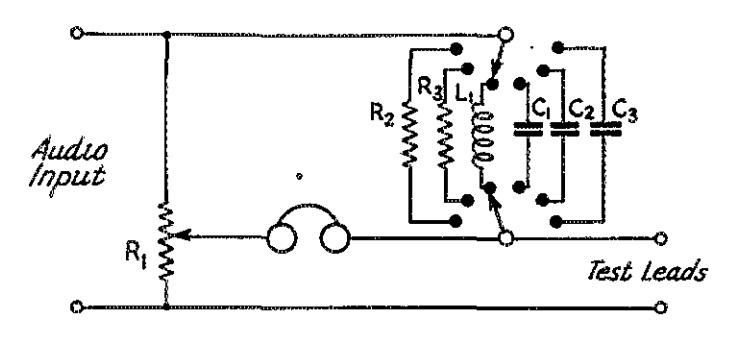
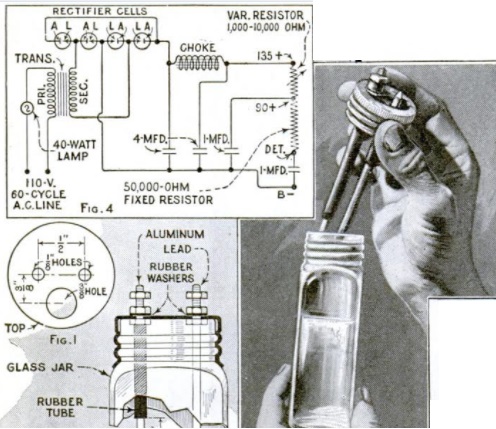
 If you’re reading this page because Junior just remembered that the science fair project needs to be handed in tomorrow, there’s no need to panic! This site is full of interesting science fair projects. Some of those projects might take some time to complete, but many of them can be whipped into shape in an evening. This one falls into that category. Junior can make an impressive display by mapping magnetic lines of force.
If you’re reading this page because Junior just remembered that the science fair project needs to be handed in tomorrow, there’s no need to panic! This site is full of interesting science fair projects. Some of those projects might take some time to complete, but many of them can be whipped into shape in an evening. This one falls into that category. Junior can make an impressive display by mapping magnetic lines of force.
If the project is due tomorrow, have Junior start reading the Wikipedia article about magnetic lines of force. In the meantime, you can race to the hardware store or dollar store to get the items he’ll need, if you don’t have them already.
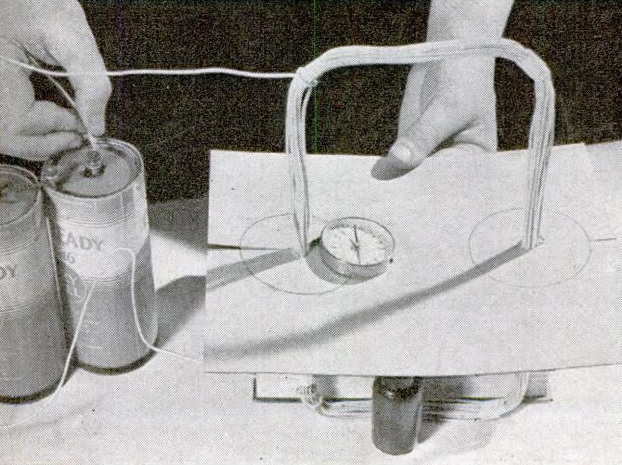
First, you’ll need some insulated wire. Any kind of wire will work, as long as it’s insulated. At the dollar store, you can probably find it in the electronics aisle in the form of speaker wire. Get about 10-20 feet.
You’ll also need a battery. The old-fashioned dry cells shown above look nice, but there’s really no need. Normal D-cell flashlight batteries will work just fine. Get a few extras, since they will be used up quickly.
Finally, you’ll need a cheap compass. If you don’t have one already, you can probably find one in the toy department, and it will work fine. And while you’re there, don’t forget to get the obligatory poster board, markers, etc.
Have junior construct the loop of wire shown in the picture, and embed it in a piece of cardboard. Hook the ends of the wire to the battery, and you have created an electromagnet. Simply move the compass around the cardboard, and with a pencil, make a small mark showing what direction the compass is pointing. Connect all of these marks, and you will have an accurate diagram of the magnetic lines of force.
The teacher will be impressed, Junior will get a blue ribbon, and nobody will know that he waited until the last minute.
You can find more details in the March 1943 issue of Popular Science, from which the illustration was taken.
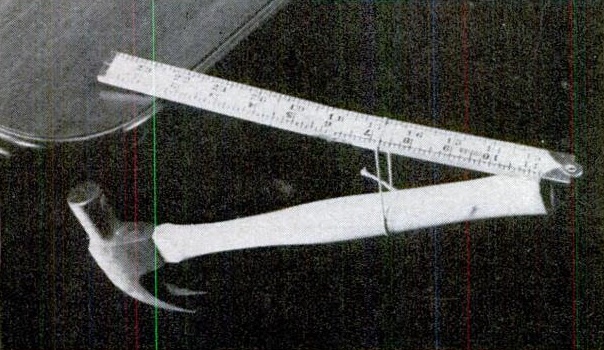 Seventy-five years ago this month, the June 1943 issue of Popular Science showed how to do this seemingly impossible balancing act. The hammer is suspended by a string in the middle, with the end of the handle exerting an upward force on the end of the ruler. Since the center of gravity is under the table, the ruler stays in place.
Seventy-five years ago this month, the June 1943 issue of Popular Science showed how to do this seemingly impossible balancing act. The hammer is suspended by a string in the middle, with the end of the handle exerting an upward force on the end of the ruler. Since the center of gravity is under the table, the ruler stays in place.