Eighty years ago, there was a war going on, but that didn’t stop young scientists on the home front from conducting experiments with the items available. If Junior’s science fair project is due tomorrow, he can take a cue, and whip together a great project from these ideas in the December 1942 issue of Popular Science.
Eighty years ago, there was a war going on, but that didn’t stop young scientists on the home front from conducting experiments with the items available. If Junior’s science fair project is due tomorrow, he can take a cue, and whip together a great project from these ideas in the December 1942 issue of Popular Science.
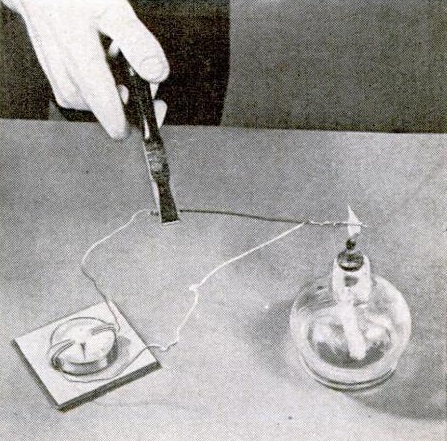
Both experiments involve a galvanometer, and the magazine presupposes that young scientists know how to make one, because the instructions merely state to use “your homemade galvanometer.” But in case Junior doesn’t know, it’s easy enough. You just take a compass (a toy one from the dollar store will work just fine) and wrap a few turns of wire around it. The smallest of electric currents will cause the compass to move. If there’s a little extra time before the science fair, we’ve previously shown how to make a deluxe galvanometer.
In the first experiment, shown above, Junior can demonstrate that water, contrary to popular opinion, is an insulator. It’s only when a little bit of salt is added to the water that it starts to conduct electricity, and the compass moves. To accentuate the effect, we recommend starting with distilled water. And don’t worry about not being able to find the old-fashioned dry cell batteries. These experiments will work just as well with modern alkaline D cells, especially if you purchase battery holders for them.
The second experiment, shown below, shows how to make a thermocouple. Two pieces of wire–copper and iron (or steel)–are twisted together. When the twisted wires are put into a flame of a candle, the dissimilar metals produce an electric current, as shown by a movement of the compass. And as we always remind parents about experiments involving flames, don’t forget to give Junior a box of matches!
As with many of our projects, Junior is almost guaranteed to take home the blue ribbon, and the teacher won’t have a clue that the project was put off until the last minute.
Some links on this site are affiliate links, meaning this site earns a small commission if you make a purchase after using the link.