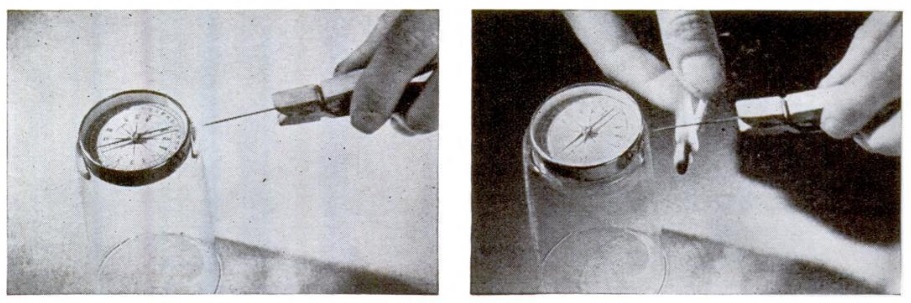
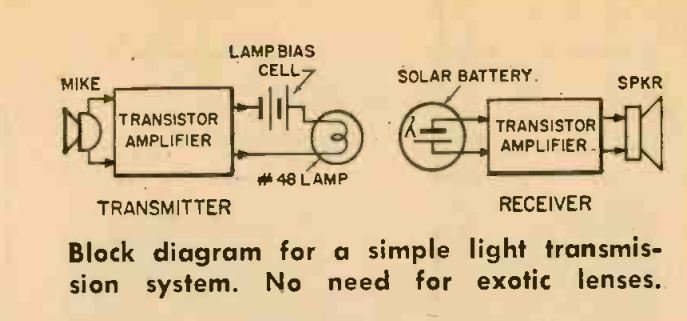
Seventy-five years ago this month, the March 1951 issue of Popular Science showed how to put together a science fair project that was, for many years, quite popular. You can make your own cloud chamber, which allows you to watch cosmic rays and the decay of atomic particles. It’s rather easy to make. You make a supersaturated layer of alcohol vapor, which is done with the use of dry ice. The only other materials needed can be found around the house.
Seventy-five years ago this month, the March 1951 issue of Popular Science showed how to put together a science fair project that was, for many years, quite popular. You can make your own cloud chamber, which allows you to watch cosmic rays and the decay of atomic particles. It’s rather easy to make. You make a supersaturated layer of alcohol vapor, which is done with the use of dry ice. The only other materials needed can be found around the house.
The alcohol can be ethyl, methyl, or isopropyl. If Junior is taking it to school, it’s probably best to avoid ethyl alcohol. In addition, that kind (in the form of “Everclear” most likely) is more expensive than isopropyl, which can be had at any drug store.
Dry Ice can be found at the better convenience stores in your area. Of course, use caution when handling it. Actually, you shouldn’t handle it at all. Bring an insulated container to the store and have them place it in there.