 Every self-respecting young mad scientist probably discovered as a toddler that many hours of fun could be had by mixing vinegar and baking soda. When the time comes for their first science fair in elementary school, many of them harken back to this early experiment and decide to make a vinegar and baking soda volcano. In fact, if you Google the words “science fair vinegar and baking soda volcano“, you will get over a million results, many of which we are sure are fine educational projects. But we also know that our readers, even the youngest ones, strive for a bit more. So if your young mad scientist is thinking of a science fair project involving these venerable home chemicals, here are a couple of more advanced projects, which appeared in Boys’ Life magazine 70 years ago this month, March 1951. They are just as easy (or even easier) than the stereotypical volcano that other kids will be bringing, but they demonstrate some additional scientific principles.
Every self-respecting young mad scientist probably discovered as a toddler that many hours of fun could be had by mixing vinegar and baking soda. When the time comes for their first science fair in elementary school, many of them harken back to this early experiment and decide to make a vinegar and baking soda volcano. In fact, if you Google the words “science fair vinegar and baking soda volcano“, you will get over a million results, many of which we are sure are fine educational projects. But we also know that our readers, even the youngest ones, strive for a bit more. So if your young mad scientist is thinking of a science fair project involving these venerable home chemicals, here are a couple of more advanced projects, which appeared in Boys’ Life magazine 70 years ago this month, March 1951. They are just as easy (or even easier) than the stereotypical volcano that other kids will be bringing, but they demonstrate some additional scientific principles.
To do the experiment shown above, you start with a tall glass of water and add a tablespoon of vinegar. You then slowly stir in a half teaspoon of baking soda. Finally, you put four or five mothballs in the glass. They’re heavier than water and will sink to the bottom, but after about a minute, they will rise to the surface. They’ll sink again and continue rising and falling for hours.
What’s happening is that the mothballs are only slightly heavier than water. Little carbon dioxide bubbles affix themselves to the surface, which gives just enough buoyancy for them to rise. At the surface, the bubbles pop, and the mothball sinks again. If you don’t have any mothballs around the house, I’m told that raisins will work just as well.
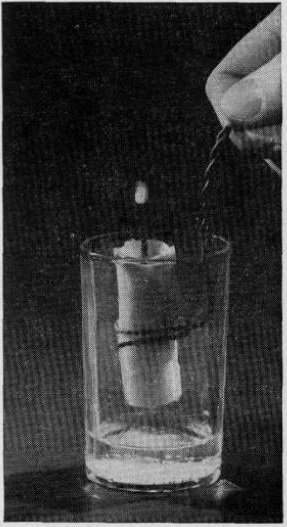 If Junior likes playing with fire (and what kid doesn’t?), they will enjoy the experiment shown at the left. You start with a teaspoon of baking soda at the bottom of an empty glass. To this, you add a tablespoon of vinegar diluted with a tablespoon of water. When the mixture begins to fizz, you lower a lighted candle into the glass. Since carbon dioxide is heavier than air, it fills the glass. And since carbon dioxide doesn’t support a flame (which is why fire extinguishers use it), the flame extinguishes itself.
If Junior likes playing with fire (and what kid doesn’t?), they will enjoy the experiment shown at the left. You start with a teaspoon of baking soda at the bottom of an empty glass. To this, you add a tablespoon of vinegar diluted with a tablespoon of water. When the mixture begins to fizz, you lower a lighted candle into the glass. Since carbon dioxide is heavier than air, it fills the glass. And since carbon dioxide doesn’t support a flame (which is why fire extinguishers use it), the flame extinguishes itself.
But there’s more! If you act quickly enough, you can lift the candle back out as soon as it goes out, and the flame will miraculously come back to life. This is because there is still vaporized wax, and the wick is still hot enough to ignite as soon as it gets back into sufficient oxygen.
The kid who made the volcano will undoubtedly go home with a nice participation ribbon. But armed with these simple experiments proving scientific principles, Junior will undoubtedly take home the blue ribbon.

