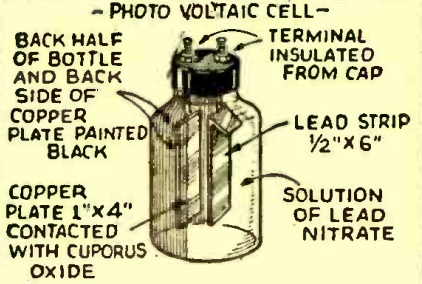
 Seventy-five years ago this month, the February 1943 issue of Radio Craft showed how to make this rudimentary photovoltaic cell.
Seventy-five years ago this month, the February 1943 issue of Radio Craft showed how to make this rudimentary photovoltaic cell.
With some modification, it scould be easily duplicated today, and could be the basis for an interesting science fair project.
It consists of a strip of lead, as well as a copper plate covered with cuporus oxide. To achieve the coating, the copper plate is heated in a flame until it is covered by a black flaky substance, which is cupric oxide. Then, it is washed in a weak solution of ammonia, which reveals the light-sensitive cuprous oxide.
A sheet of lead should be available at a craft store, or can be ordered from Amazon. Similarly, a small piece of copper is readily available at a hardware store or Amazon.
The electrolyte is a somewhat more difficult proposition. The lead nitrate is somewhat hazardous, but should be safe if handled carefully. The main problem is that it is expensive. It is available on Amazon, both as a solution and as crystals, However, the prices might be outside the young mad scientist’s budget.
Fortunately, this site seems to suggest that ordinary salt water will function adequately as the electrolyte. Therefore, one suitable science fair project might be to determine what other electrolyte solutions might work best. All that would be required would be a voltmeter to see which configuration puts out the most electricity. The advanced student could skip buying the voltmeter and instead make this simple galvanometer.
Another fun project would be to demonstrate communication over a light beam, with a setup similar the one on this site. Your homemade photocell is hooked to the input of a small audio amplifier, and you hook an LED to the headphone jack of a radio or other audio source.
