If you’re looking for a good inexpensive flashlight, I recommend the modern one shown at the left. For just a few dollars, you get a reliable light with a long lasting battery, and replacement batteries will only set you back a few cents.
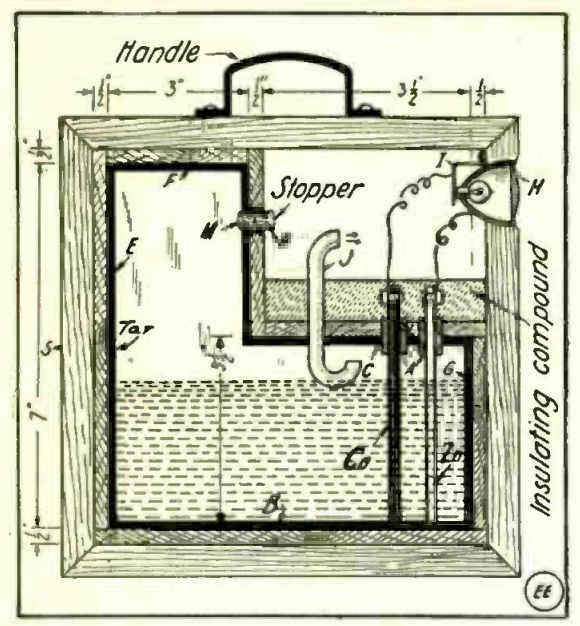
But a hundred years ago, while handheld flashlights were available, they were more expensive and less reliable. So the economy minded handyman might consider the idea of just making his own, battery and all. The self-explanatory diagram shown at the top of the page, taken from the December 1916 issue of Electrical Experimenter, shows exactly how it can be done. The great advantage of this do-it-yourself model was that it didn’t even require a switch. You just set the light on its side, all of the electrolyte would slosh to the compartment on the left, and the light would extinguish.
The lamp is built from nice thick pieces of hardwood, which securely contain the acid sloshing around inside. What could possibly go wrong? Well, what could go wrong is hinted at by this admonition in the instructions:
The next, and perhaps the most important step, to be taken in the construction of the portable battery lamp, is to seal up the battery jar so as to make it absolutely acid-proof. To prevent the acid from leaking out of the jar the cracks can be filled up by applying a thin coat of molten pitch or asphalt around the inside walls of the jar, as indicated by the heavy black line. This can be done very well by melting a few pounds of asphalt in a kettle and pouring a small quantity through the rubber tube holes at A, tilting the whole case so that it will run into all the corners; then permit it to cool. Additional molten tar is added until every crevice and surface of the interior is thoroughly coated with the insulating and acid-proof material.
After this acid-proof container is thus constructed, the interior is filled with a solution of sulfuric acid. When the lamp is set as shown in the diagram, the bulb should glow brightly.
This is presented as inspiration for a science fair project, but even I would recommend that aspiring scientists contemplating a similar project should make a few more concessions to safety. The school janitor probably won’t be very pleased if some sulfuric acid leaks out of your wooden “jar” onto the floor of the school gymnasium. And mom probably won’t appreciate it if you melt a few pounds of asphalt in one of her kettles. But the general concept is sound. It’s quite possible to make your own battery capable of generating honest to goodness electrical current. For more ideas on other homemade batteries, you can visit my earlier post on the subject. You can find other science fair ideas at this link.

