Eighty-five years ago, Britain was at war, and that meant shortages of many things, including flashlight batteries. Undaunted, many Britons took to making their own, and the February 1940 issue of Practical Mechanics showed them how to do it.
 There was a learning curve involved, but the magazine assured readers that the task was well within the capabilities of amateurs. The costs of materials were low, and once you were set up, you could laugh at the Nazis trying to deprive you of batteries.
There was a learning curve involved, but the magazine assured readers that the task was well within the capabilities of amateurs. The costs of materials were low, and once you were set up, you could laugh at the Nazis trying to deprive you of batteries.
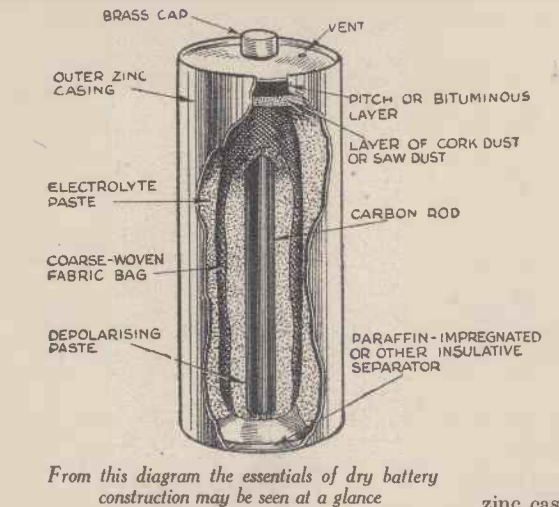
The article pointed out that filling the cells, at least initially, was a messy process. But once you got into a routine, it was relatively easy. The article suggested getting together a quantity of zinc containers and carbon rods, and then commencing the filling process. Surrounding the carbon rod was a “depolarizing paste” consisting of a mixture of approximately equal quantities of carbon or plumbago powder and manganese dioxide (pyrolusite) made into a paste with a 1 per cent. solution of gum tragacanth. The electrolyte consisted of about 85 per cent. of plaster of Paris and 15 per cent. of ordinary flour mixed to a just-wet paste with a strong solution of sal ammoniac,
For the student looking for a science fair project, making a battery is always a worthwhile option. In addition to this set of instructions, we have many other similar ideas on this site.
Some links on this site are affiliate links, meaning that this site earns a small commission if you make a purchase after using the link.
