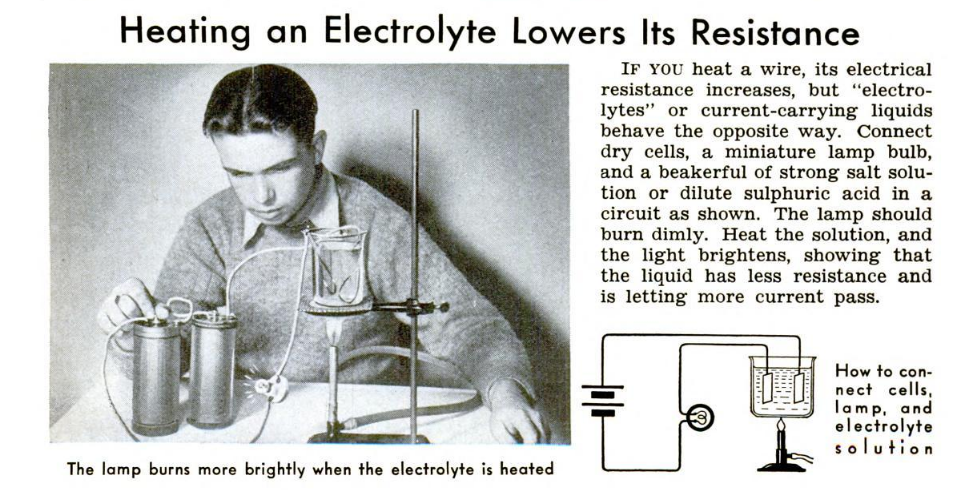
 If you’re looking for an interesting science fair project, this one from the August 1939 issue of Popular Science is interesting, looks like you put a lot of work into it, but is really quite simple. It answers the scientific question, “does the temperature of an electrolyte solution affect the conductivity?” It turns out, as this experiment will show, that the answer is yes. As the temperature increases, so does the conductivity. (Or to put it another way, as the temperature goes up, the resistance goes down.)
If you’re looking for an interesting science fair project, this one from the August 1939 issue of Popular Science is interesting, looks like you put a lot of work into it, but is really quite simple. It answers the scientific question, “does the temperature of an electrolyte solution affect the conductivity?” It turns out, as this experiment will show, that the answer is yes. As the temperature increases, so does the conductivity. (Or to put it another way, as the temperature goes up, the resistance goes down.)
The experiment, as shown above, is relatively simple. You should be able to find all of the required supplies locally (if you don’t already have them at home). If you want to order online, the links below are to Amazon.
The 1939 version of the experiment shows old fashioned dry cell batteries, but modern alkaline D cell batteries will work just fine. You can figure out some other way to attach the wires to the batteries, but life is a lot easier if you use a battery holder.
Any flashlight bulb will work, as long as it’s from a flashlight that normally uses 2 batteries. This one is suitable. Again, you can figure out some other way to connect the wires, but having a socket will make things easier. Finally, for the electric hookups, you will need some kind of wire, although almost any will work just fine.
The two electrodes going into the solution can be almost anything metallic. I would recommend using some large nails.
You’ll need a beaker and some lab hardware to support it while heating, although if you should be able to borrow that from your science teacher. If you’re doing the experiment in the school lab, you can use a bunsen burner. If you’re doing it elsewhere, you’ll need a heat source such as an alcohol burner.
The experiment calls for either salt water or sulfuric acid. You surely have salt at home. If you prefer to go with sulfuric acid, you can order that online, or ask for it in the hardware store, where it’s available as a drain cleaner.
